Abstract
Introduction
Despite the availability of novel agents in treating multiple myeloma (MM), renal impairment (RI) remains a poor prognostic factor, and the median survival of patients (pts) with MM and RI is approximately half of that for MM pts with normal renal function. RI can affect up to 50% of pts with MM at presentation, highlighting the need for effective treatment options for this patient population. Daratumumab, an IgG1 κ human monoclonal antibody that targets CD38, has shown efficacy and a favorable safety profile in pts with relapsed or refractory MM (RRMM). The DARE study assessed the safety and efficacy of daratumumab with dexamethasone in pts with RRMM and severe RI or requiring hemodialysis.
Methods
DARE is a prospective, open-label, phase 2 study, conducted in eight sites in Greece and Italy. Eligible pts were adults with documented RRMM and severe RI (estimated glomerular filtration rate [eGFR]<30 ml/min/1.73m 2) or requiring hemodialysis, who have had ≥2 prior lines of therapy (including bortezomib- and lenalidomide-based regimens), progressive disease by International Myeloma Working Group (IMWG) criteria, and an Eastern Cooperative Oncology Group performance status (ECOG PS) score ≤2. Exclusion criteria included previous treatment with anti-CD38 antibodies, including daratumumab. Pts received 28-day treatment cycles with 16mg/kg intravenous daratumumab (weekly for cycles 1-2, every 2 weeks for cycles 3-6, and every 4 weeks thereafter) and oral dexamethasone (40mg weekly, each cycle). The primary endpoint was progression-free survival (PFS). Secondary endpoints included the overall response rate (ORR; proportion of pts with partial response or better), the renal response rate (RRR; proportion of pts with best response of renal partial response or better), and the safety of daratumumab. All responses were assessed by IMWG criteria.
Results
The study has completed accrual and 38 pts were enrolled. The pts' median (range) age was 72 (40-89) years, and most pts were male (29, 76.3%). At baseline, 37 (97.4%) pts had ECOG PS ≤1 and 34 (89.5%) were at International Staging System (ISS) stage III. By revised ISS, 20 (52.6%) and 16 (42.1%) pts were at stages II and III, respectively. Pts had a median (range) of 3 (2-6) prior systemic therapies; thirteen (34.2%) pts had undergone prior autologous stem cell transplantation. The median eGFR at baseline was 13.0 mL/min/1.73m 2 and seventeen (44.7%) pts were on dialysis at the time of enrollment.
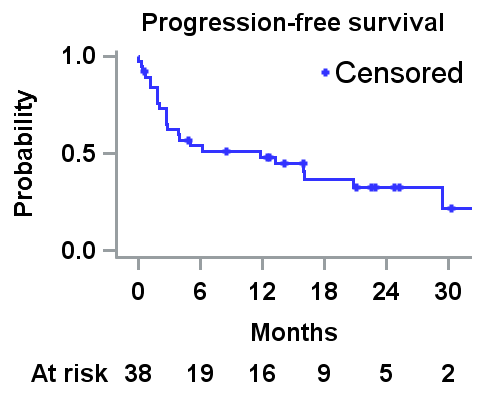
The median (range) number of cycles given was 8.0 (1.0-38.0), and the median (range) follow-up was 11.3 (<0.1-36.0) months. The median PFS is 11.8 (95% confidence interval [CI]: 2.8-20.8) months (Figure). The ORR was 47.4% with 13 (34.2%) and 5 (13.2%) pts achieving a very good partial response (VGPR) and a partial response (PR), respectively. The ORR for dialysis pts was 47.1% with 5 (29.4%) and 3 (17.6%) pts achieving VGPR and PR, respectively. The median (95%CI) time to first response (≥PR) was 0.9 (0.9-9.6) months. The RRR was 18.4% (5.9% for dialysis pts and 28.6% for non-dialysis pts). The median (95%CI) OS was 24.5 (5.5-NR) months, and the median (95%CI) DOR (≥PR) was 28.4 (15.1-NR) months; the median (95%CI) OS for pts on dialysis and non-dialysis was 12.5 (2.2-NR) and 24.5 (10.1-NR0) months, respectively. The median (95%CI) DOR for pts on dialysis was not reached (15.1-NR) months and for non-dialysis pts was 28.4 (3.5-NR) months respectively.
Overall, 19 (50.0%) pts had ≥1 grade 3/4 adverse event (AE), and 10 (26.3%) had ≥1 serious AE (SAE). The most common grade 3/4 AEs were anemia (6 pts, 15.8%), hyperglycemia (5 pts, 13.2%), and hypercalcemia (3 pts, 7.9%). The most common SAE was septic shock (3 pts, 7.9%).
Conclusions
The administration of daratumumab with dexamethasone in pts with RRMM and severe RI or requiring hemodialysis is safe and effective therapy associated with a median PFS of approximately 12 months; hematologic responses were rapid and observed within one month from treatment initiation and approximately one-fifth of pts achieved a major renal response. Almost half of the pts were on dialysis and the combination was active and safe also for those on dialysis. No new safety signals were observed with daratumumab in pts with RRMM and severe RI or in need of dialysis.
Kastritis: Janssen: Consultancy, Honoraria, Research Funding; Takeda: Honoraria; Pfizer: Consultancy, Honoraria, Research Funding; Genesis Pharma: Honoraria; Amgen: Consultancy, Honoraria, Research Funding. Terpos: Sanofi: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria, Research Funding; Novartis: Honoraria; Janssen-Cilag: Consultancy, Honoraria, Research Funding; GSK: Honoraria, Research Funding; Genesis: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria, Research Funding; BMS: Honoraria; Amgen: Consultancy, Honoraria, Research Funding. Symeonidis: Demo: Research Funding; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; WinMedica: Research Funding; Pfizer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; GenesisPharma: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Gilead: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; GSK: Research Funding; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; MSD: Consultancy, Research Funding; Sanofi/Genzyme: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Astellas: Consultancy, Research Funding; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Delimpasi: Janssen: Honoraria, Speakers Bureau; Amgen: Honoraria, Speakers Bureau; Takeda: Honoraria, Speakers Bureau. Cavo: Bristol-Myers Squib: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: TRAVEL, ACCOMMODATIONS, EXPENSES, Speakers Bureau; Sanofi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; GlaxoSmithKline: Consultancy, Honoraria; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Adaptive Biotechnologies: Consultancy, Honoraria; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Accommodations, Speakers Bureau; Novartis: Honoraria. Zamagni: Janssen: Honoraria; Bristol-Myers-Squibb: Honoraria; Takeda: Honoraria; Amgen: Honoraria. Katodritou: GSK, Amgen, Karyopharm, Abbvie, Janssen-Cilag, Genesis Pharma, Sanofi: Honoraria, Research Funding. Kyrtsonis: Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene/Genesis Pharma: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Honoraria; Sanofi: Membership on an entity's Board of Directors or advisory committees. Hatjiharissi: Gilead: Honoraria; Genesis: Honoraria, Research Funding; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees. Leonidakis: Health Data Specialists: Current Employment. Manousou: Health Data Specialists: Current Employment. Gavriatopoulou: Sanofi: Honoraria; Genesis: Honoraria; Amgen: Honoraria; Janssen: Honoraria; GSK: Honoraria; Karyopharm: Honoraria; Takeda: Honoraria. Dimopoulos: Amgen: Honoraria; Beigene: Honoraria; Janssen: Honoraria; BMS: Honoraria; Takeda: Honoraria.